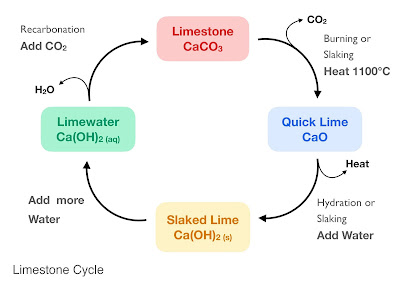
Limestone Cycle
Limestone cycle is one of the prominent examples of chemistry nature has given birth to. It comprises of limestone (calcium carbonate), lime (calcium oxide), slaked lime (anhydrous calcium hydroxide) and limewater (aqueous calcium hydroxide). Each of these have different and important uses, thus are interconverted for the required purpose.
The cycle starts with calcium carbonate. Calcium Carbonate when heated gives off Carbon Dioxide and produces Calcium Oxide (Quick Lime).
CaCO3 + heat → CaO + CO2
When we add water to Calcium Oxide it gives us anhydrous Calcium Hydroxide if the amount of water and Calcium Oxide is taken proportionately.
CaO + H2O → Ca(OH)2 + heat
And when we add more water to Anhydrous Calcium Oxide we get Hydrated Calcium oxide or Limewater.
Finally if you pass carbon dioxide though Limewater it will again produce Limestone and water, thus completing the cycle.
Ca(OH)2 + CO2 → CaCO3 + H2O
However if we pass excess carbon dioxide through lime water the Carbon Dioxide reacts with water and formed Calcium Carbonate to give Calcium Hydrogen Carbonate.
CaCO3 + CO2 + H2O → Ca(HCO3)2
The above figure gives a quick glans of the limestone cycle. Each of its components are described elaborately below.
Limestone
Limestone is type of sedimentary rock with high concentration of calcium carbonate.
Chemical Properties
- Chemical Name: Calcium Carbonate
- Chemical Formula: CaCO3
- Molar Mass: 100.09 g/mol
- Melting Point: 825°C (aragonite)
Formation
Millions of years ago the skeleton and shells of many dead aquatic organisms deposited to the seabed, over years these deposits have become thicker, compression by upper sedimentary layers leads to the formation of limestone.
Synthesis
Calcium Carbonate can be synthesised by recarbonisation of limewater.
When Carbon Dioxide is passed through lime water we get Calcium Carbonate and Water.
Ca(OH)2 + CO2 → CaCO3 + H2O
Uses
Limestone is used as a building material in the form of Marble. Taj Mahal, one the the seven wonders of the world is made of Marble only.
Quick Lime
Quick lime or burnt lime or Calcium Oxide is one the most widely used chemical compound.
Chemical Properties
- Chemical Name: Calcium Oxide
- Chemical Formula: CaO
- Molar Mass: 56.0774 g/mol
- Melting Point: 2,613 °C
Occurrence
Quick Lime occurs naturally as mineral named Lime
Synthesis
Calcium oxide can be synthesised by calcination of limestone.
When heated above 1100°C calcium carbonate reduces to calcium oxide with release of carbon dioxide as a byproduct. The reaction for thermal decomposition of limestone:
CaCO3 + heat → CaO + CO2
Uses
- Cement Industry: Calcium oxide is largely used in making of cement
- Food Additive: FAO recognises Calcium Oxide as an antacid and for a lot many uses
- Glass Industry: widely used in the process of making glass
- As water softeners
- Preparing bleaching powder
Slaked Lime
Slaked lime is the anhydrous form of calcium hydroxide.
Chemical Properties
- Chemical Name: Anhydrous Calcium Hydroxide
- Chemical Formula: Ca(OH) (s)
- Molar Mass: 74.09 g/mol
- Melting Point: 579.85 °C
Synthesis
Slaked Lime or Calcium Hydroxide does not occur naturally. It can be synthesised by slaking of Calcium Oxide.When water is added to Calcium Oxide it produces Calcium Hydroxide along with a large amount of heat. Calcium Hydroxide is not very soluble in water thus it precipitates. This can then be extracted by Drying or a Vacuum filter.
CaO + H2O → Ca(OH)2 + heat
Uses
- Paper Industry
- Used in pickle
- Used in Paan and tabacco
- Antacid
Limewater
Limewater is the anhydrous form of calcium hydroxide and have same chemical properties as Slaked Lime.
Synthesis
To prepare it just add water to the slaked lime
Uses
- Chemistry: To test presence of carbon dioxide
- Used in softening of water
- Chemistry: To test presence of carbon dioxide
- Used in softening of water